Using DNA from archival specimens to study the origins and evolution of HIV: implications for the design of more effective vaccines
Team members involved: Melanie Kuch and Debi Poinar
Previous team members involved: Michael Bunce, John Okello
Project also in collaboration with Ken Rosenthal
Funding provided by: CIHR (Canadian Institutes of Health Research)
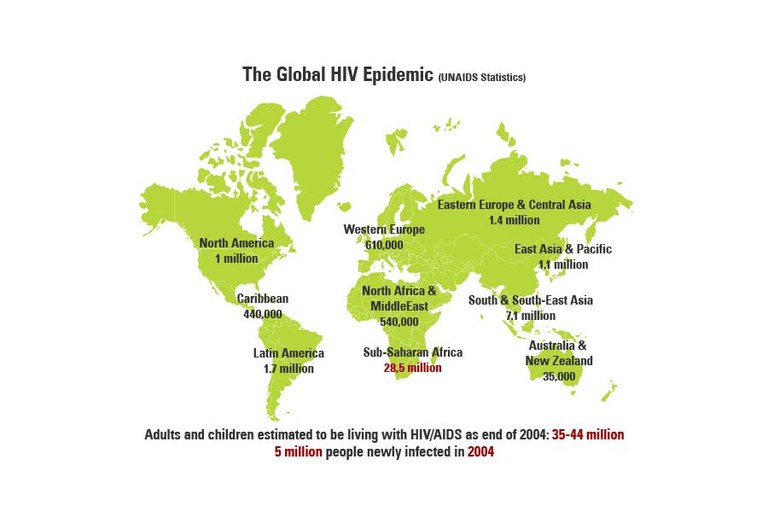
Sub-Saharan Africa is home to more than two-thirds of the world’’s people living with HIV/AIDS.
Central Africa, specifically the Democratic of the Congo, is also the only region in the world where all HIV-1 Groups, M, N and O, and their subtypes, exist. The closest viral match to HIV-1 is a simian immunodeficiency virus, SIVcpz, harbored in the chimpanzee sub-species, Pan troglodytes troglodytes, which lives throughout equatorial Western and Central Africa. It is suspected that sometime prior to 1959 (the earliest confirmed case of HIV-1) a zoonotic event took place when an SIV crossed into the human population via the chimpanzee, resulting in the emergence of HIV-1.
The mystery remains; Exactly when, where, why and how did this pathogen come to infect the human population?
The Archival HIV Project hopes to answer these essential questions by reconstructing the past through an extensive molecular analysis of ancestral strains of HIV-1 found in archival samples in conjunction with an in-depth study on the history of Africa from 1890 to mid 1980. Historical journals, books, medical documents and literature will be utilized to construct a comprehensive epidemiological and socioecological picture of Africa before and during the purposed time period of HIV-1 emergence.
A parallel project involving the analysis of chimpanzee faces to discover novel SIV sequences that may be more closely related to HIV-1 is also an integral part of the Archival HIV Project.
Over the past few years we have collected a variety of archival tissues samples from Central Africa which may harbor DNA/RNA from HIV (and other pathogens). While there is no possibility that any infectious virus has survived – trace amounts of its genetic material will still be present in the samples. Our Lab uses “ancient DNA” techniques to recover and repair trace amounts of genetic material and then use PCR (a technique by which to “photocopy” DNA) to generate sufficient quantities of DNA to sequence the virus.
Examples of archival tissues include serum, blood slides, wax embedded autopsy blocks, electrophoresis smears, alcohol-stored biopsies (see figure). The DNA and RNA in many of these samples is present in very small quantities and can be highly degraded – The technical challenge is to tease-out as much information as possible from this material.
HIV is one of the fastest mutating organisms known to date. The vast majority of HIV sequence data comes from isolates that have been characterized in the past 20 years. Very few sequences exist prior to 1980 – this “gap” represents a void in our knowledge of what the earliest forms of HIV looked like and how it “altered” the first few years after it made the “jump” from primates into humans.
The ultimate aim of this project is to incorporate information obtained from “fossil” viral sequences into vaccines. Due to the extremely fast mutation rate of HIV many vaccines have proven ineffective, they simply cannot afford protection across multiple HIV subtypes. In collaboration with Dr Ken Rosenthal we will test how archival HIV sequences (T and B-cell epitopes) protect against viral challenge. Our hope is that ancestral sequences will represent an effective means by which to generate good immunological protection across a wide variety of HIV variants.
Map of the Congo – Archival tissues have been sourced from across the region.
The Congo is believed to be the site of the first HIV infections.
A selection of archival samples to be screened for HIV
Top left: wax embedded lung tissue from young boy from Kinshasa who likely died of HIV in 1982 – but who probably contracted the virus pre-natal in 1974.
Bottom left: serum from a Congolese long-term non-progressor that has been sampled throughout the infection which originally occurred in the 1970’s.
Right: Post-mortem lung biopsy of a female Danish surgeon from Yambuku who died of HIV-like symptoms in 1977.